Myasthenia gravis (MG) is a rare autoimmune disease in which the body’s defense system attacks the connection point between nerves and muscles. This can cause double vision, drooping of the eyelids, and difficultly swallowing, talking, breathing, and using the arms and legs.
Clinical Trials

Investigational Study Drug
Combinations of different investigational study drugs
Study Status
Start up
Clinical Study Code
ARGX-999-2-MG-2000
Clinicaltrials.Gov Number
NCT07294170
About MG
About this clinical research study
Adapt Forward is a platform of studies designed for people living with MG. It consists of several studies that are meant to evaluate how different combinations and regimens of MG drugs may work (also known as efficacy) and how safe they are for the use in adults living with MG.
Different investigational study drugs or combinations of different study drugs are evaluated in the platform studies. In some studies, the study drug is compared to a placebo and in others it’s compared to other MG study drugs. Different regimens are evaluated in the different studies.
It is important to understand that these study drugs/combinations have not been approved by health authorities for the use(s) being studied.
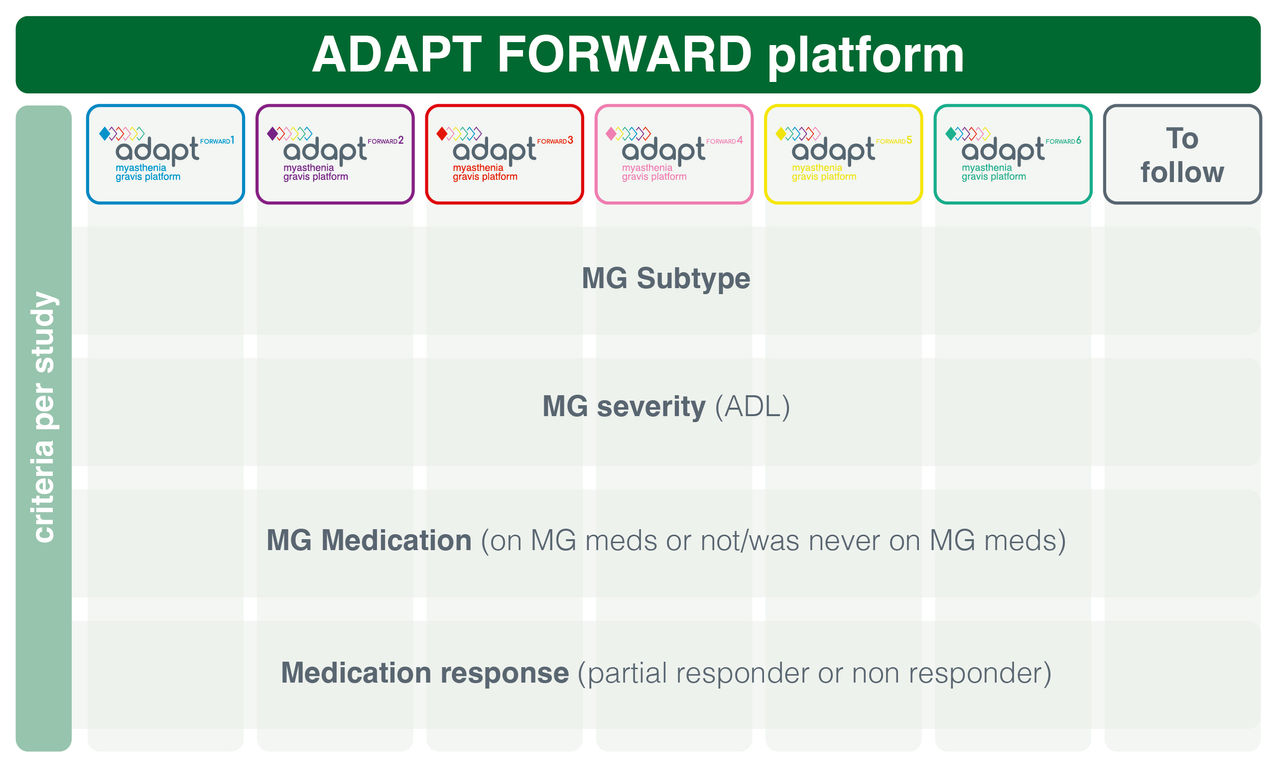
Click on below logos to find more information about each study.
Eligibility (Age, condition)
You may be eligible to participate if you are:
- At least 18 years old
- Not pregnant, or actively trying to get pregnant
- Diagnosed with MG
Additional eligibility criteria per study apply.
What to expect
A study consists of below phases:
Master protocol screening: If interested in joining the platform, a screening process to check eligibility will be initiated. This includes laboratory tests, a physical examination, review of medical history and MG medication history, and completing questionnaires. If qualified to participate in the Platform, the results will be reviewed to determine which studies running under this platform may be best and a more detailed check will be done to find out further eligibility.
Study screening: A more detailed check to find out if the potential participant is eligible for a study.
Treatment phase: If eligible to join a study, the participant will be assigned to receive the investigational study drug(s) in accordance to the study regimen of the assigned study.
Follow up phase: After the treatment phase, the study team will continue to monitor for safety.
Study locations
The clinical study sites are located across the world. Discover open study sites on the map.
Discover Locations for This Study
- All


